Penicillin (Benzylpenicillin)
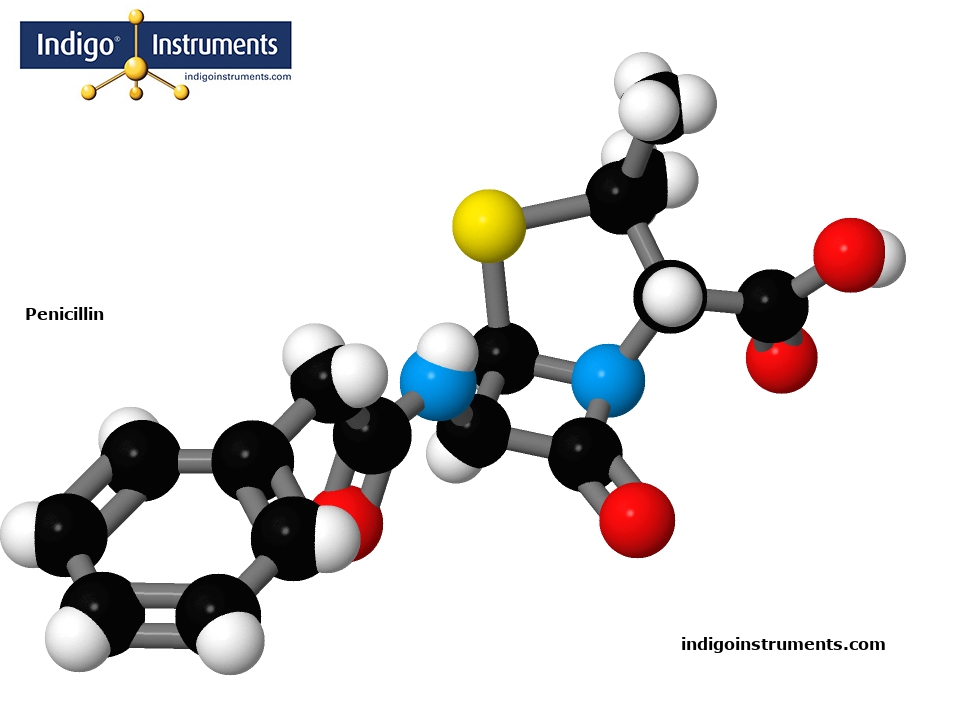
Construct a 3D molecule model of antibiotic Penicillin using Authentic Molymod Components by Indigo.
Penicillin G (benzylpenicillin), was the first antibiotic found to be an effective for treating Gram-positive bacteria such as Streptococcus sp. & Staphylococcus sp.
All penicillins share a common molecular structure, a beta-lactam ( β-lactam ) ring, a thiazolidine ring, and a side chain, 6-aminopenicillanic acid. Penicillin G’s mode of action iinterferes with the integrity of bacterial cell walls by inhibiting the enzyme which cross-links peptidoglycan, the key structural component.
There are several natural penicillins (penicillin dihydro F, X, and K) but penicillin G is the most active and the only natural form used in clinical applications.& The differences in the penicillins are in the side chains which prevent bacterial enzymes from hydrolysing the beta-lactam ring.
A molecular model of Penicillin in the Molymod Hybrid Dome style uses the atoms & bonds in the parts list below. Our free 3D Molecular Model Builder shows other styles & allows for image rotation as an assembly guide.
| Molecular Weight | Chemical Formula |
|---|---|
| 357.379 | C16H18N2NaO4S |