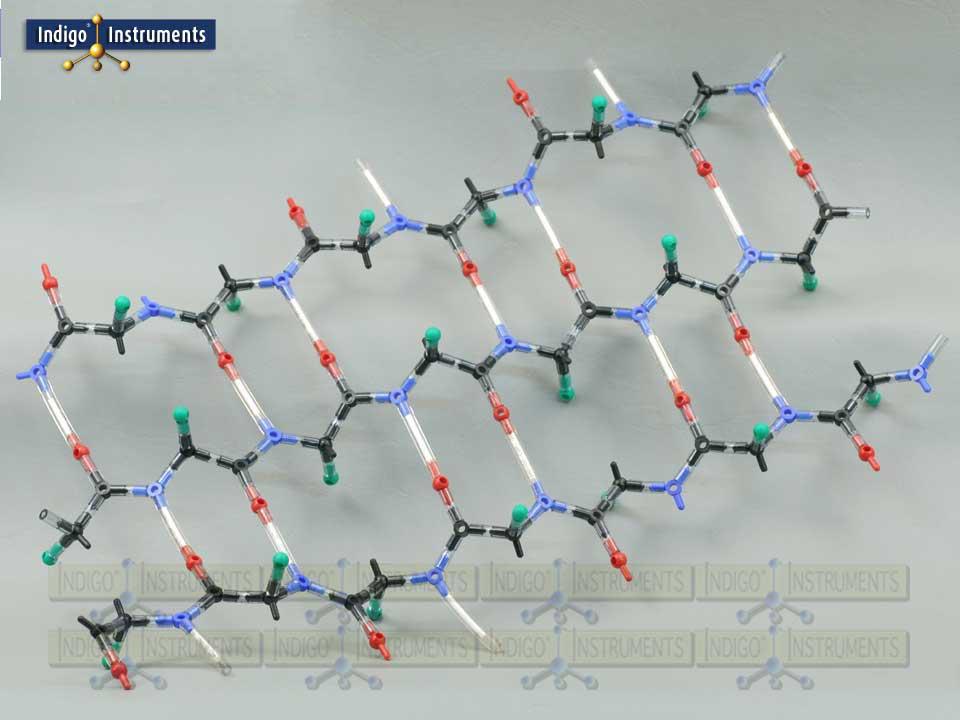
Alpha Helix Protein Structure Model
SKU: 68796W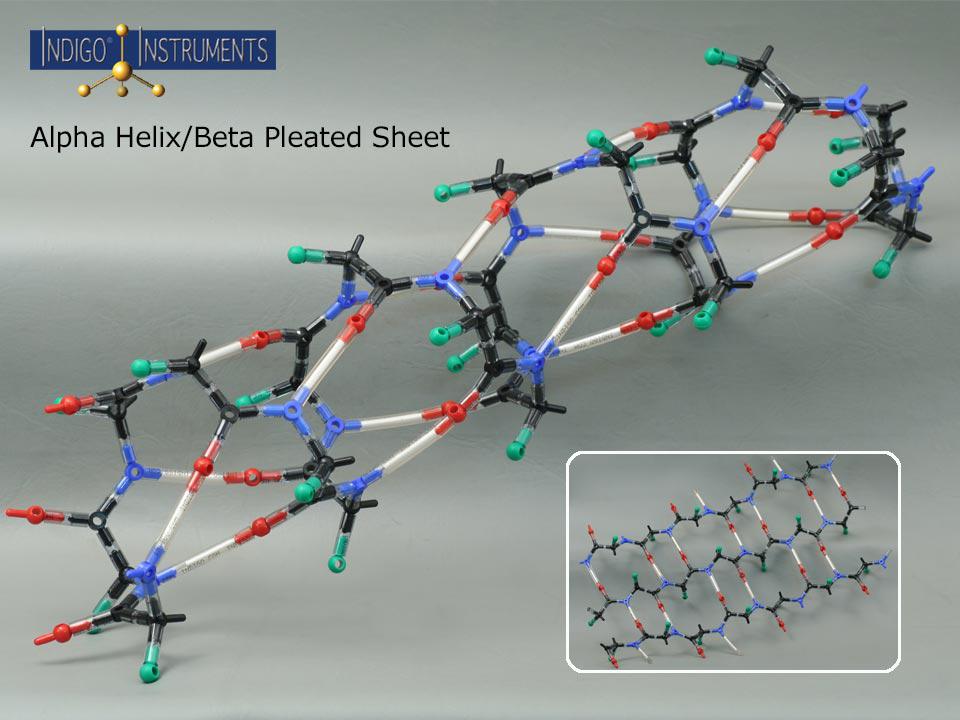
This Indigo® molecular model kit can build an alpha helix or beta sheet to explore hydrogen bonding, sidechain orientation and more.
This molecular model kit provides a tangible way to visualize hydrogen bonding, backbone geometry, and sidechain orientation. The alpha helix model is sized to fit naturally into the major groove making it an ideal companion to our 12-base-pair Indigo® DNA double helix model. By manipulating the model, learners can explore how amino acid sequence drives structure and gain a hands-on understanding of protein folding, stability, and interactions
“R” groups are indicated with a green "atoms" but parts that represent actual sidechains can be added. The 50mm bonds with white inserts represent the amide hydrogen atoms (N–H) of residues that form hydrogen bonds with the carbonyl oxygen atoms (C=O) of another residue. In alpha helices, these bonds stabilize the coiled structure with the characteristic i → i+4 pattern, while in beta sheets, hydrogen bonds form between strands in either parallel or antiparallel arrangements. This makes it easier to visualize how hydrogen bonding contributes to the stability and geometry of both secondary structure types.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.