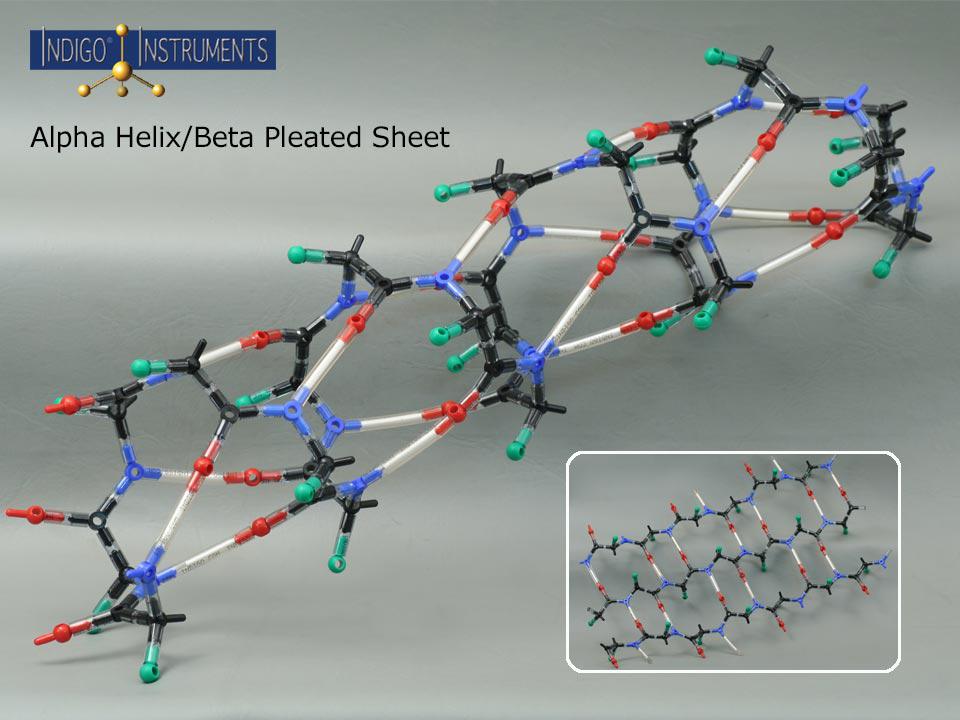
Beta Pleated Sheet
SKU: 68796W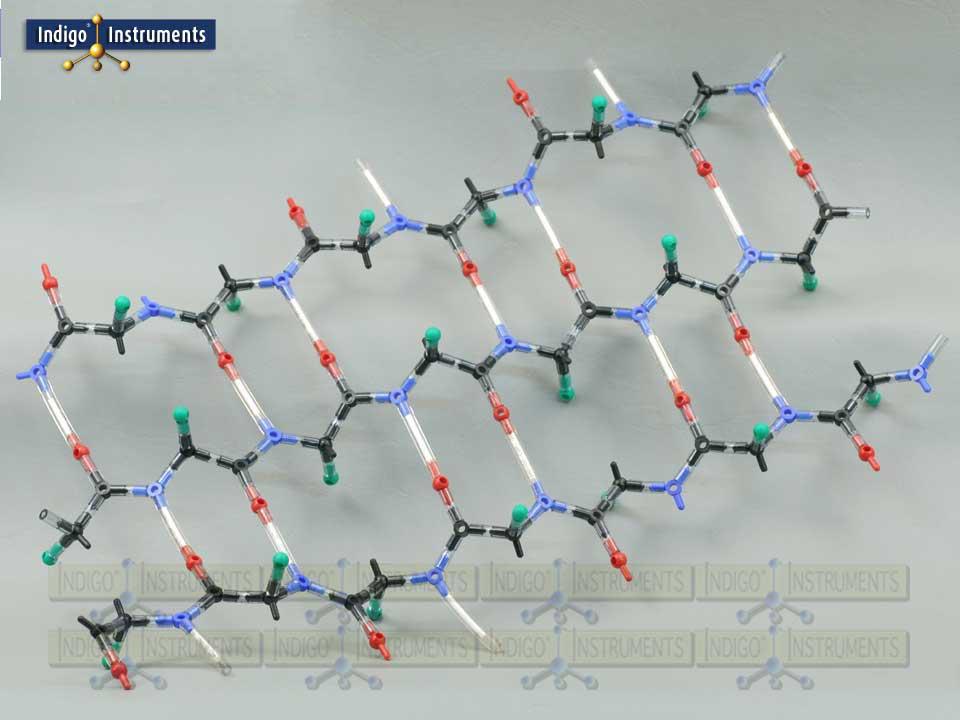
This molecular model kit can build the backbone of a beta pleated sheet using 20 amino acid cores. The model consists of alpha carbons and amino & carboxyl groups with "R" groups projecting above & below the sheet's plane. The sheet can be built with chains running parallel or antiparallel where the N & C terminal ends alternate. You will need two kits to compare beta sheet structures with parallel & antiparallel versions .
In parallel beta sheets all the polypeptide strands run in the same direction (from N-terminus to C-terminus). Antiparallel beta sheets run in opposite directions, where one strand goes from N-terminus to C-terminus and the next goes from C-terminus to N-terminus. This is also reflected in hydrogen bonding strength which is less in parallel than in antiparallel sheets (as shown in the image).
Spider & other silks are characterized in having large sections of antiparallel beta sheet structures.
Indigo Instruments has maintained a substantial inventory of genuine Cochranes of Oxford (Orbit) parts for 30+ years (scroll down to see "Skeletal (Orbit/Minit) and are compatible with every molecular model kit we have sold since day 1. This level of quality may appear expensive but no parts support from other vendors costs even more.