C2v Cyclohexane Boat
SKU: 68821W
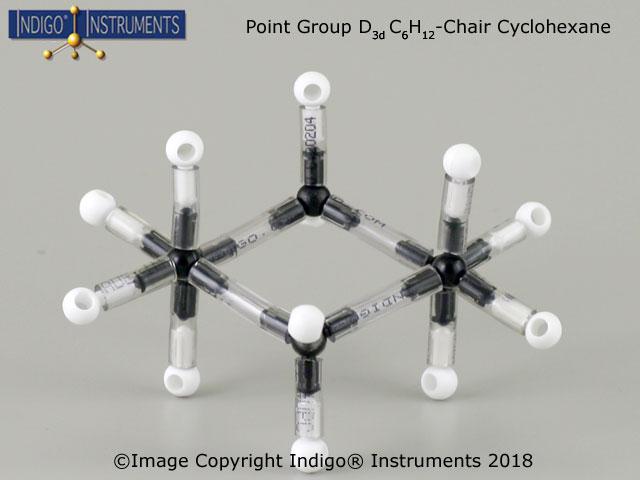
The Indigo® Instruments 68821W molecular model kit comes with parts to build several structures illustrating C2v point group symmetry including cyclohexane.
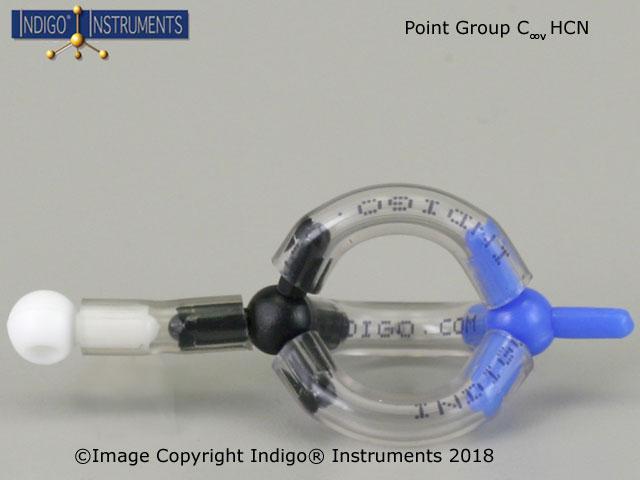
A molecule’s symmetry, such as C2vC_{2v}C2v, simpliifies the complexity of molecular orbital calculations such as symmetry-adapted linear combinations (SALCs) & aids chemists in applying group theory to predict molecular vibrations, electronic transitions, and bonding characteristics.

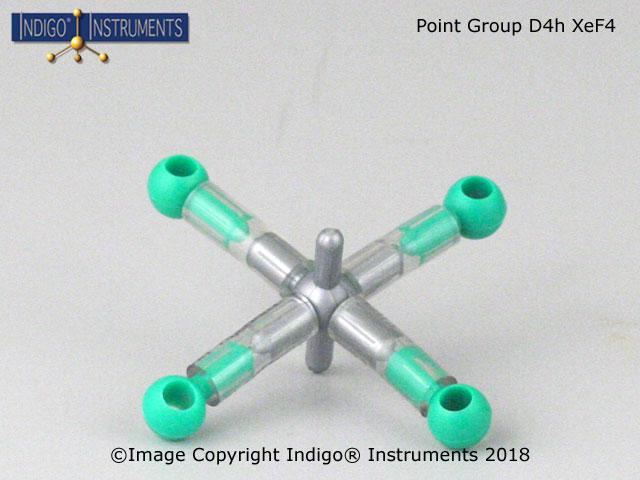


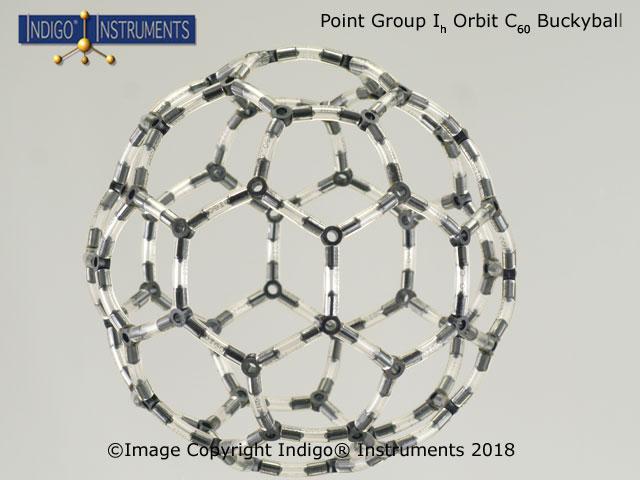





![D3 [Co(en)3]3+ D3 [Co(en)3]3+](/images/products-resp/point-group-d3-68821w.jpg)












Thanks for the feedback. It is an unusual set & the only one we know of that can build ferrocene.