VSEPR Octahedral Geometry
SKU: 69180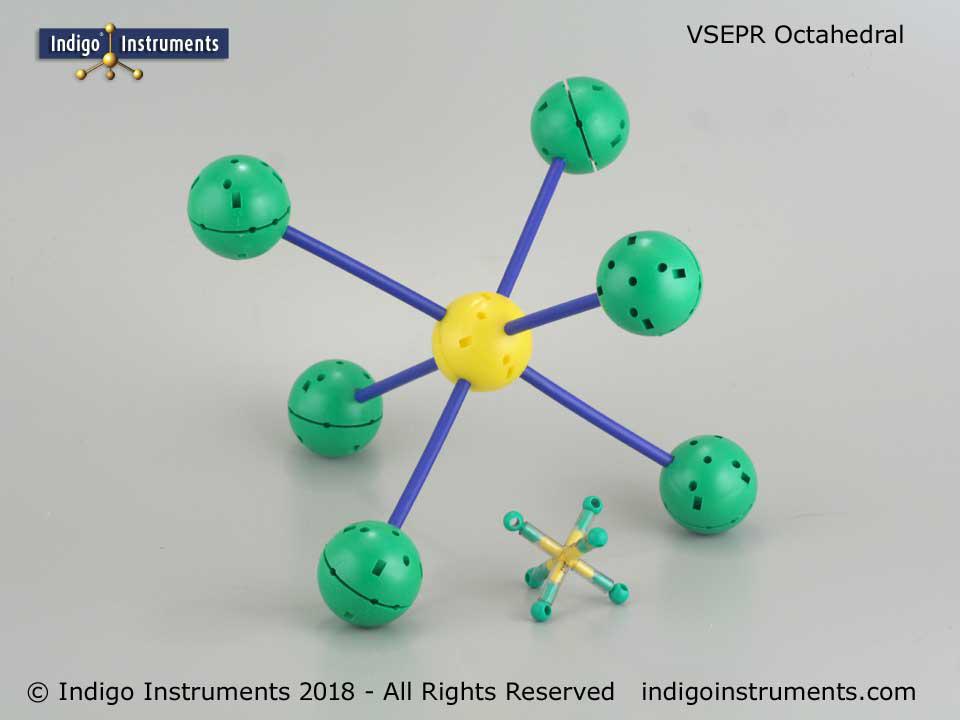
SF6 (suflur hexafluoride) is a molecule representative of octahedral bond angle geometry & this model is roughly 300mm (12") end to end when constructed with Unit model parts.
Every atom in this VSEPR Classroom Model set can assume any geometry. Go to the bottom of page Unit Molecular Models for an overview or click on the Instructions/Safety tab to see videos on how to construct any molecular geometry.
You can also see an example of octahedral geometry in our student VSEPR theory models & compare model sizes to this Unit Octahedral Model. You can also see how it would look in our 62009 student organic/inorganic chemistry model set.
SF6 molecular geometry consists of 4 equally spaced planar atoms at right angles to 2 atoms in a configuration as predicted by VSEPR theory with an AX6E0 molecular shape designation.
SF6 has a centrally located sulfur atom surrounded by 6 fluorine atoms. This molecular geometry has an octahedral shape. Further, SF6 is nonpolar since the 6 symmetrically positioned fluorine atoms cancel out the bond dipoles. Consequently, SF6 is not water soluble but can be dissolved in non-polar organic solvents.


Thanks for the feedback. It can indeed be a bit tedious setting up the various hole configurations but that is also the beauty of it. No other set is as versatile in creating both standard VSEPR geometries and ones only found in crystals.